It seems we can’t find what you’re looking for. Perhaps searching can help.
Category:
Top Articles
-
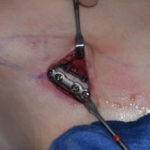
Clinical Outcomes in Rib Removal Surgery for Waistline Reduction
Rib removal surgery represents the elimination of the last anatomic...
-
The Surgical Technique of Clavicular Osteotomies in Shoulder Width Reduction
Shoulder width reduction is done by shortening the length of the...
-
Masculinizing the Male Forehead with Custom Brow Bone Implants
The shape and appearance of the forehead is highly influenced by...
-
Case Study: Lengthening of the Nose with a Rib Graft Rhinoplasty
Background: The evolution of rhinoplasty surgery over the past twenty years...
Nothing Found
Top Articles
-
Clinical Outcomes in Rib Removal Surgery for Waistline Reduction
Rib removal surgery represents the elimination of the last anatomic...
-
The Surgical Technique of Clavicular Osteotomies in Shoulder Width Reduction
Shoulder width reduction is done by shortening the length of the...
-
Masculinizing the Male Forehead with Custom Brow Bone Implants
The shape and appearance of the forehead is highly influenced by...
-
Case Study: Lengthening of the Nose with a Rib Graft Rhinoplasty
Background: The evolution of rhinoplasty surgery over the past twenty years...